FSMA
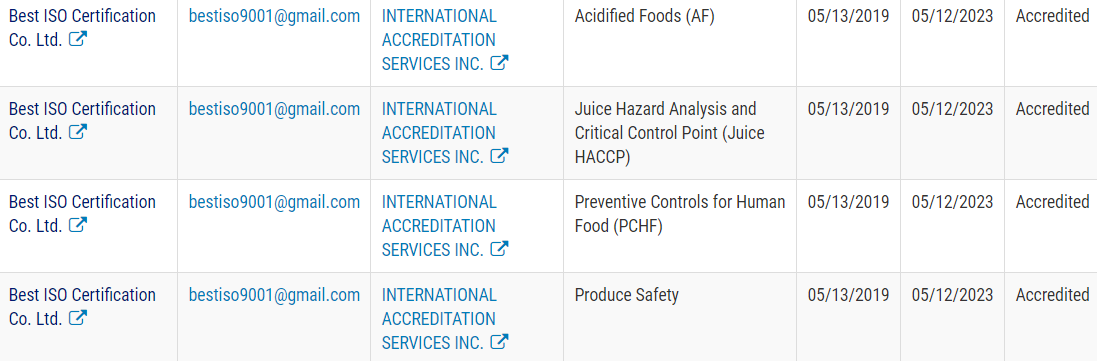
Best ISO got the First FDA Accredited Third-Party Accredited Certification Program: Public Registry of Accredited Third-Party Certification Bodies in Asia
FDA Food Safety Modernization Act
Overview
Under the FDA recognition, IAS has the authority to accredit third-party certification bodies, also known as third-party auditors. Best ISO Certification. Co. Ltd (Best ISO) is the pilot certification bodies, once accredited, can conduct food safety audits and issue certifications of foreign food facilities (including farms) and the foods – both human and animal – that they produce. Those certifications can be used by importers to establish eligibility for participation in the Voluntary Qualified Importer Program (VQIP), and in certain circumstances FDA can require that imported products be certified before entering the United States.
Under the FDA recognition, IAS has the authority to accredit third-party certification bodies, also known as third-party auditors. Best ISO Certification. Co. Ltd (Best ISO) is the pilot certification bodies, once accredited, can conduct food safety audits and issue certifications of foreign food facilities (including farms) and the foods – both human and animal – that they produce. Those certifications can be used by importers to establish eligibility for participation in the Voluntary Qualified Importer Program (VQIP), and in certain circumstances FDA can require that imported products be certified before entering the United States.
Introduction for FDA FSMA and VQIP
This document sets forth the requirements for obtaining and maintaining International Accreditation Service, Inc. (IAS), Third-party Certification Bodies (Best-ISO Certification) under the Food & Drug Administration (FDA) Food Safety Modernization Act (FSMA) accreditation and for the qualifying data that must be submitted relating to the scope of Certification. The Third-party Certification clients seeking accreditation for this accreditation program shall comply with the requirements specified in Federal Register Vol.80, No. 228, dated November 27, 2015, by FDA; and supplemented by this IAS program requirement, IAS Rules of Procedure for Third- party Certification Body under the Food & Drug Administration (FDA) Food Safety Modernization Act (FSMA), and International Accreditation Forum (IAF) guidance documents on certification or application of Management System Standards and this Rule
The Voluntary Qualified Importer Program (VQIP) is a voluntary fee-based program that provides expedited review and import entry of human and animal foods into the United States for participating importers. Both consumers and importers will benefit from this program.
Participating importers will be able to import their products to the U.S. with greater speed and predictability, avoiding unexpected delays at the point of import entry. Consumers will also benefit from the importer’s robust management of the safety and security of their supply chains.
To participate, importers must meet eligibility criteria and pay a user fee that covers cost associated with the FDA’s administration of the program.
The U.S. Food and Drug Administration (FDA) will be opening the Voluntary Qualified Importer Program (VQIP) application portal on October 1, 2018. This will allow importers to submit their completed applications early for the FY20 benefit year. Importers interested in applying can start their application by submitting a notice of intent to participate by setting up an account via the FDA Industry Systems website. Once you have an account, selecting VQIP under the FSMA Program options will take you to the VQIP Application Page with an option for submitting a Notice of Intent to Participate. Importers applying for the FY20 benefit period may wish to refer to the Step-by-Step guide as they prepare their applications.
Annual user fees to participate in VQIP cover FDA’s costs for administering the program. These include the costs of reviewing applications; the costs of conducting inspections of importers (both foreign and domestic) accepted into the program; and the annual Information Technology (IT) maintenance costs. The user fee rates are calculated each Fiscal Year and will be posted in a Federal Register notice on or before August 1 each year.
These certifications are used for two purposes.
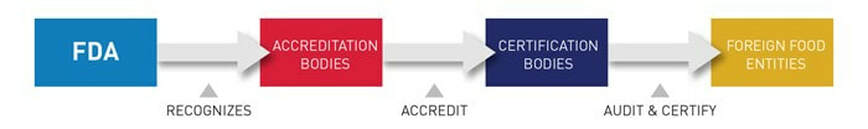
FDA’s Accredited Third-Party Certification Program was established under the FDA Food Safety Modernization Act (FSMA). It is a voluntary program that allows “accreditation bodies” to apply for recognition by FDA. Recognized accreditation bodies will have the authority to accredit third-party Certification Bodies.
Application
1.0 All clients need to delivery the following application information before the audit scheduling.
2.0 And understanding the following requirements for the special BEST ISO-IAS-FDA FSMA requirement.
This legally enforceable agreement include provisions to ensure it can be extended until all transfer activities to the new FDA-recognized certification body are completed
The contract between the certification body and the client shall address the following items:
a) the client shall notify the certification body of any changes (see section 3.2),
b) the client cannot refuse an FDA & IAS witness audit of the certification body,
c) the client cannot refuse the presence of a certification body internal witness auditor,
d) the client cannot refuse the presence of an FDA & IAS representative or their delegates,
e) the client cannot refuse the request of the certification body to provide the final report to the FDA & IAS,
f) the only use of the FDA & IAS logo related to this certification scheme is as displayed on the certificate issued by the certification body. Any other use of the FDA & IAS logo, separately or not, is prohibited,
g) consultants to the client cannot be physically present at the client’s site during the audit or participate in the audit in any way
h) the client shall pay all fees in advance and that no any return.
i) The certification is maxim 12 months from the issued day. Certificate cycle : The recertification decision shall be made before the expiration date of the existing certificate. The recertification decision date shall be the issue date of the new certificate
J) The English audit report need to delivery to IAS and FDA
This document sets forth the requirements for obtaining and maintaining International Accreditation Service, Inc. (IAS), Third-party Certification Bodies (Best-ISO Certification) under the Food & Drug Administration (FDA) Food Safety Modernization Act (FSMA) accreditation and for the qualifying data that must be submitted relating to the scope of Certification. The Third-party Certification clients seeking accreditation for this accreditation program shall comply with the requirements specified in Federal Register Vol.80, No. 228, dated November 27, 2015, by FDA; and supplemented by this IAS program requirement, IAS Rules of Procedure for Third- party Certification Body under the Food & Drug Administration (FDA) Food Safety Modernization Act (FSMA), and International Accreditation Forum (IAF) guidance documents on certification or application of Management System Standards and this Rule
The Voluntary Qualified Importer Program (VQIP) is a voluntary fee-based program that provides expedited review and import entry of human and animal foods into the United States for participating importers. Both consumers and importers will benefit from this program.
Participating importers will be able to import their products to the U.S. with greater speed and predictability, avoiding unexpected delays at the point of import entry. Consumers will also benefit from the importer’s robust management of the safety and security of their supply chains.
To participate, importers must meet eligibility criteria and pay a user fee that covers cost associated with the FDA’s administration of the program.
The U.S. Food and Drug Administration (FDA) will be opening the Voluntary Qualified Importer Program (VQIP) application portal on October 1, 2018. This will allow importers to submit their completed applications early for the FY20 benefit year. Importers interested in applying can start their application by submitting a notice of intent to participate by setting up an account via the FDA Industry Systems website. Once you have an account, selecting VQIP under the FSMA Program options will take you to the VQIP Application Page with an option for submitting a Notice of Intent to Participate. Importers applying for the FY20 benefit period may wish to refer to the Step-by-Step guide as they prepare their applications.
Annual user fees to participate in VQIP cover FDA’s costs for administering the program. These include the costs of reviewing applications; the costs of conducting inspections of importers (both foreign and domestic) accepted into the program; and the annual Information Technology (IT) maintenance costs. The user fee rates are calculated each Fiscal Year and will be posted in a Federal Register notice on or before August 1 each year.
These certifications are used for two purposes.
- Certifications can establish eligibility for participation in the Voluntary Qualified Importer Program (VQIP), which offers expedited review and entry of food.
- In rare and specific circumstances FDA can require that an imported product be certified to prevent a potentially harmful food from entering the U.S.
- To address potential safety issues before the food reaches the United States; and
- To help ensure that imported foods are produced in accordance with the same safety standards as those required of U.S. foods.
- Assess third-party certification bodies to determine if they can be accredited. This includes observing a representative sample of the applicant’s work;
- Monitor the performance of the certification bodies it accredits. Accreditation bodies must notify FDA of any change in, or withdrawal of, accreditations it has granted;
- Assess and correct any problems in the accreditation body’s own performance;
- Submit monitoring and self-assessment reports and other notifications to FDA;
- Maintain and provide FDA with access to the records that the program requires.
FDA’s Accredited Third-Party Certification Program was established under the FDA Food Safety Modernization Act (FSMA). It is a voluntary program that allows “accreditation bodies” to apply for recognition by FDA. Recognized accreditation bodies will have the authority to accredit third-party Certification Bodies.
Application
1.0 All clients need to delivery the following application information before the audit scheduling.
- Contract
- Application form ( Attachment A form 1)
- Questionnaire-EH1 for FDA ( Attachment B- Form 2-8 )
- Food safety plan issued by qualified person PCQI
- Internal auditor list
- FSMA - Quality manual
- Evidence in compliance with applicable food safety requirements of the FD&C Act and FDA regulations
- The inspection reports issued by third party laboratory with that ISO/IEC 17025 certificated accredited certificate Laboratory needs to have signed an agreements with Best ISO.
- Previous FDA relative information
- Master list for the qualified suppliers or suppliers verification plan
- Previous Management review and internal audit reports
- ISO9001 with HACCP or ISO 22000 with AB logo
2.0 And understanding the following requirements for the special BEST ISO-IAS-FDA FSMA requirement.
- All the audit must be conducted without announcement during the 30-day timeframe identified.
- ST1 readiness audit to verify the system and the all that minimum man day is one man day, must be focused on determining whether the facility, its process(es), and food are in compliance with applicable food safety requirements of the FD&C Act and FDA regulations, ( attachment C)
- ST2 will follow the man day form ( attachment D) for an onsite audit for (IAS 4.10.2) examination of the facility, its process(es), and the food that results from such process(es);and where appropriate or when required by FDA, environmental or product sampling and analysis. When, for a regulatory audit, sampling and analysis is conducted, the accredited third-party certification body must use a laboratory that is accredited in accordance with paragraph of this document 1.8. above. The audit may include any other activities necessary to determine compliance with applicable food safety requirements of the FD&C Act and FDA regulations, and, for consultative audits,also includes conformance with applicable industry standards and practices. (1.12)ISO9001 with HACCP or ISO22000 (IAS-4.10.3.) The audit must be sufficiently rigorous to allow the accredited third-party CB to determine whether the eligible entity is in compliance with the applicable food safety requirements of the FD&C Act and FDA regulations, and for consultative audits, also includes conformance with applicable industry standards and practices, at the time of the audit;and for a regulatory audit, whether the eligible entity, given its food safety system and practices would be likely to remain in compliance with the applicable food safety requirements of the FD&C Act and FDA regulations for the duration of any certification issued under this accreditation program.An accredited third-party CB that identifies a deficiency requiring corrective action may verify the effectiveness of a corrective action once implemented by the eligible entity, but must not recommend or provide input to the eligible entity in identifying, selecting, or implementing the corrective action.
This legally enforceable agreement include provisions to ensure it can be extended until all transfer activities to the new FDA-recognized certification body are completed
The contract between the certification body and the client shall address the following items:
a) the client shall notify the certification body of any changes (see section 3.2),
b) the client cannot refuse an FDA & IAS witness audit of the certification body,
c) the client cannot refuse the presence of a certification body internal witness auditor,
d) the client cannot refuse the presence of an FDA & IAS representative or their delegates,
e) the client cannot refuse the request of the certification body to provide the final report to the FDA & IAS,
f) the only use of the FDA & IAS logo related to this certification scheme is as displayed on the certificate issued by the certification body. Any other use of the FDA & IAS logo, separately or not, is prohibited,
g) consultants to the client cannot be physically present at the client’s site during the audit or participate in the audit in any way
h) the client shall pay all fees in advance and that no any return.
i) The certification is maxim 12 months from the issued day. Certificate cycle : The recertification decision shall be made before the expiration date of the existing certificate. The recertification decision date shall be the issue date of the new certificate
J) The English audit report need to delivery to IAS and FDA
Documents review
- This Contract
- Application form
- Questionnaire-EH1 for FDA
- Food safety plan issued by qualified person PCQI
- Internal auditor list
- FSMA - Quality manual
- Evidence in compliance with applicable food safety requirements of the FD&C Act and FDA regulations
- The inspection reports issued by third party laboratory with that ISO/IEC 17025 certificated accredited certificate Laboratory needs to have signed an agreements with Best ISO.
- Previous FDA relative information
- Master list for the qualifier suppliers or suppliers development plan
- Previous Management review and internal audit reports
- ISO9001 with HACCP or ISO 22000 with AB logo